Zenith Chronic Kidney Disease (CKD) and High Proteinuria Clinical Study
Austin Regional Clinic physicians constantly work to improve the care given to all our patients. We are dedicated to offering unique options for our patients and helping them reach their ideal health goals. Part of this dedication involves making clinical research available. Clinical research makes it possible to discover the benefits, risks, and safety of commercially available and investigational medications.
Research standards are very strict to help make studies as safe as possible. Teams of government and research experts have already reviewed each study we conduct to minimize any study risks. Austin Regional Clinic has decided to be involved in this research study because we believe it can potentially benefit our patients.
About the Zenith CKD and High Proteinuria clinical study
AstraZeneca researchers are investigating whether the trial drug, zibotentan combined with dapagliflozin, could help treat CKD and high proteinuria.
Zibotentan taken on its own may slow the worsening of kidney function but could also cause fluid buildup in the body. Dapagliflozin may help lower the risk of fluid buildup caused by zibotentan (when they are taken together) and has been shown to help slow the worsening of kidney function (when taken on its own).
One group of participants will take zibotentan combined with dapagliflozin, and the other group will take dapagliflozin alone. The treatment each participant takes will be randomly assigned, and none of the participants, trial doctors, or staff will know what treatment each participant takes. Participants will receive their assigned treatment for at least two years.
What to expect
Treatments and procedures: The doses of zibotentan and dapagliflozin are measured in milligrams (mg). Any participant not taking a type of medication called an SGLT2i before starting this trial will take an SGLT2i for four weeks before being assigned to one of the groups below. All participants will stop taking their SGLT2i the day before being assigned to one of the groups below. Participants will be able to continue taking their other standard CKD treatments at the discretion of trial doctors.
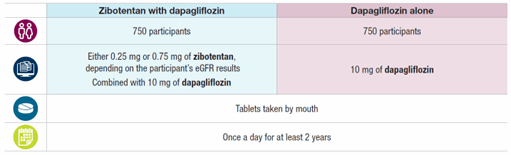
After finishing their assigned treatment, participants will take 10 mg of dapagliflozin once a day for one month. Throughout the trial, the doctors will monitor participants’ health by:
- Testing the participants’ blood and urine.
- Performing physical exams.
- Checking the participants’ vital signs.
- Testing the participants’ heart function using an electrocardiogram (ECG).
- Asking participants to fill out questionnaires about their health and quality of life.
Eligibility criteria
All participants must:
- Be 18 years of age or older.
- Be of legal age where they are participating.
- Have a current diagnosis of CKD with high proteinuria.
Participants may have type 2 diabetes but cannot join the trial if they have type 1 diabetes or other serious medical conditions.
Location
Request to participate
Please contact our ARC Clinical Research team directly to request to participate by calling 737-247-7240. If you would like research to contact you, please complete this form to request participation.